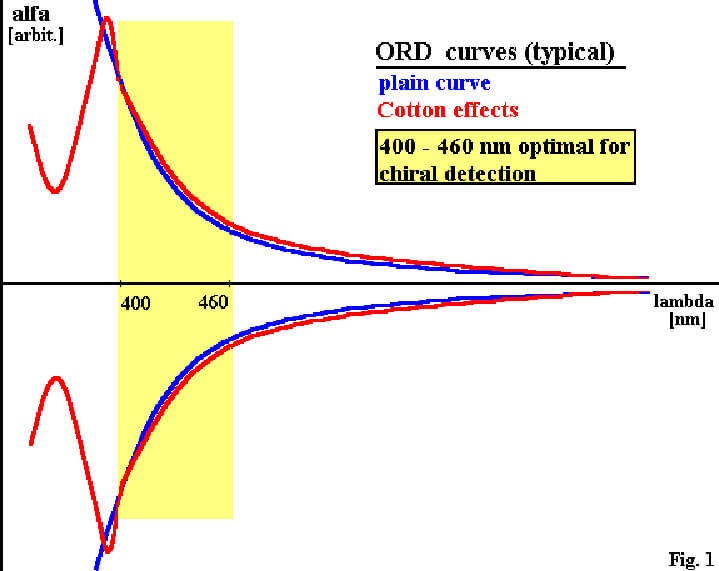
The dependence of the rotational strength optical active molecules on the wavelength is described by the Optical Rotation Dispersion (ORD). It is specific to the chiral molecule and differs in intensity, but in general, shows higher rotational strength for shorter wavelength in the so called plain curve. It is connected with Drude ́s equation and describes the normal behaviour of the ORD in absence of chromophores or far away from absorbtion band [1]
Drude ́s equation:
$$[\alpha]_{\lambda}=\sum A_i/(\lambda_2-\lambda_i)$$
(\(A_i\) constant molecular characteristic)
(\(\lambda_i\)i constant wavelength)
Within or nearby strong absorbtion band, the ORD curve may become anormal and will not obey Drude ́s equation anymore, showing Cotton effects and may even change sign of rotation. Due to this, the light spectra for chiral detection is restricted by strong absorbtion and Cotton effects for short wavelength in spite of high optical rotation. Going to longer wavelength (IR), the optical rotation becomes extremely weak and therefor gets unfit for chiral detection.
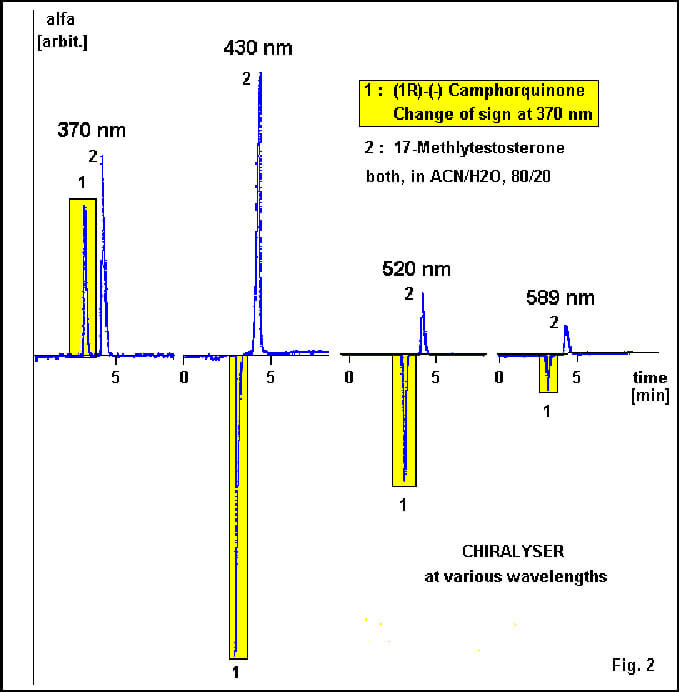
The CHIRALYSER [2,3] (IBZ MESSTECHNIK GMBH, Germany), chiral detector for monitoring optical active molecules in analytical and preparative HPLC, operates at 430 nm to profit by high optical rotation for short wavelenght without getting in conflict with absorbtion phenomenia. The light source is a high power blue LED (light emiting diode) which, in contrary to a laser, will not show the opto-physical disadvantages in chiral detection.
FIGURE 1: Typical ORD curve
FIGURE 2: (1R)-(-) Camphorquinone at different wavelength
by : W. Zelenka (Ibz Messtechnik GmbH) , P. Rozea (Synergetic Associates Inc.), J. MacFarlane (Jm Science Inc.)
References:
[1] G. Snatzke, Angew.Chemie, 80, 15ff (1968)
[2] W. Zelenka, A. Leiminer, A. Manschreck,
GIT Fachz.Lab.37 (1993); Chem.Abstr.118 (1993) 182 432
[3] IBZ (unpublished results)